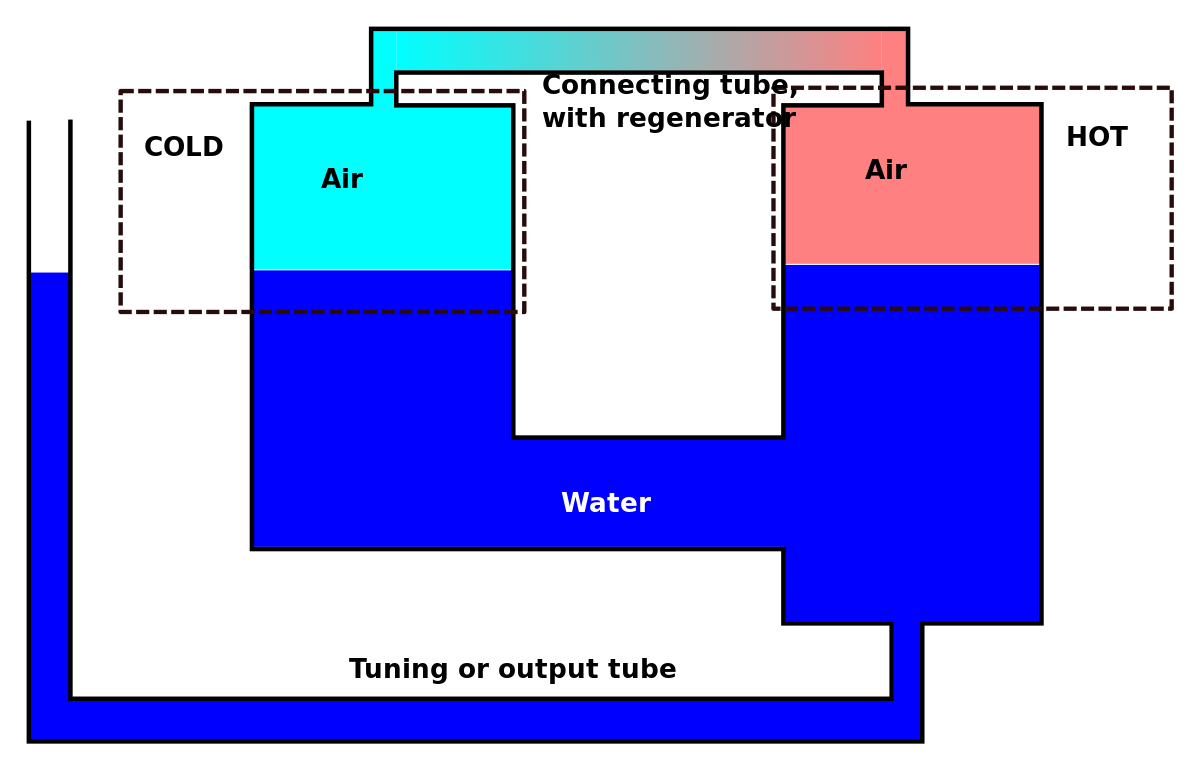
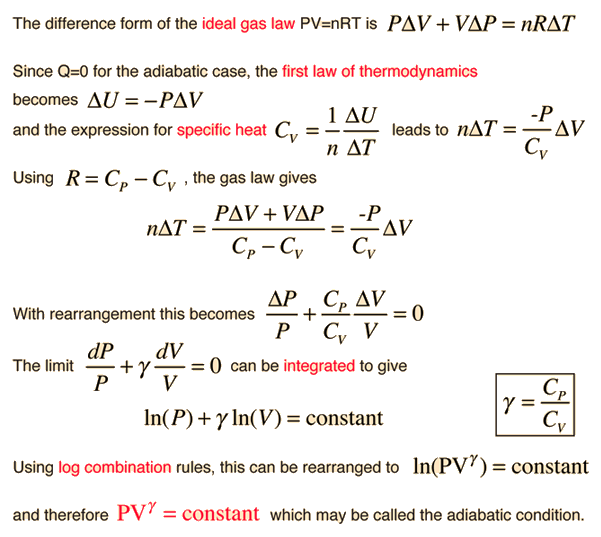
View of Factors Influencing the Thermodynamic Efficiency of Stirling Engines | PAM Review Energy Science & Technology
Energies | Free Full-Text | An Analysis Model Combining Gamma-Type Stirling Engine and Power Converter | HTML
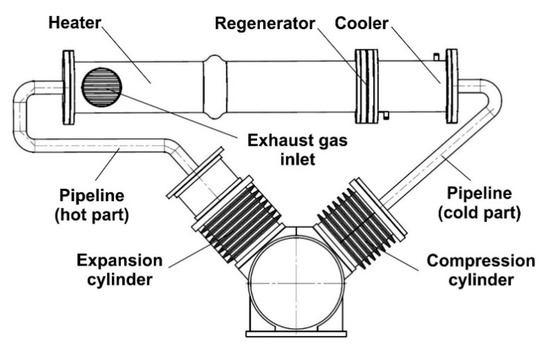
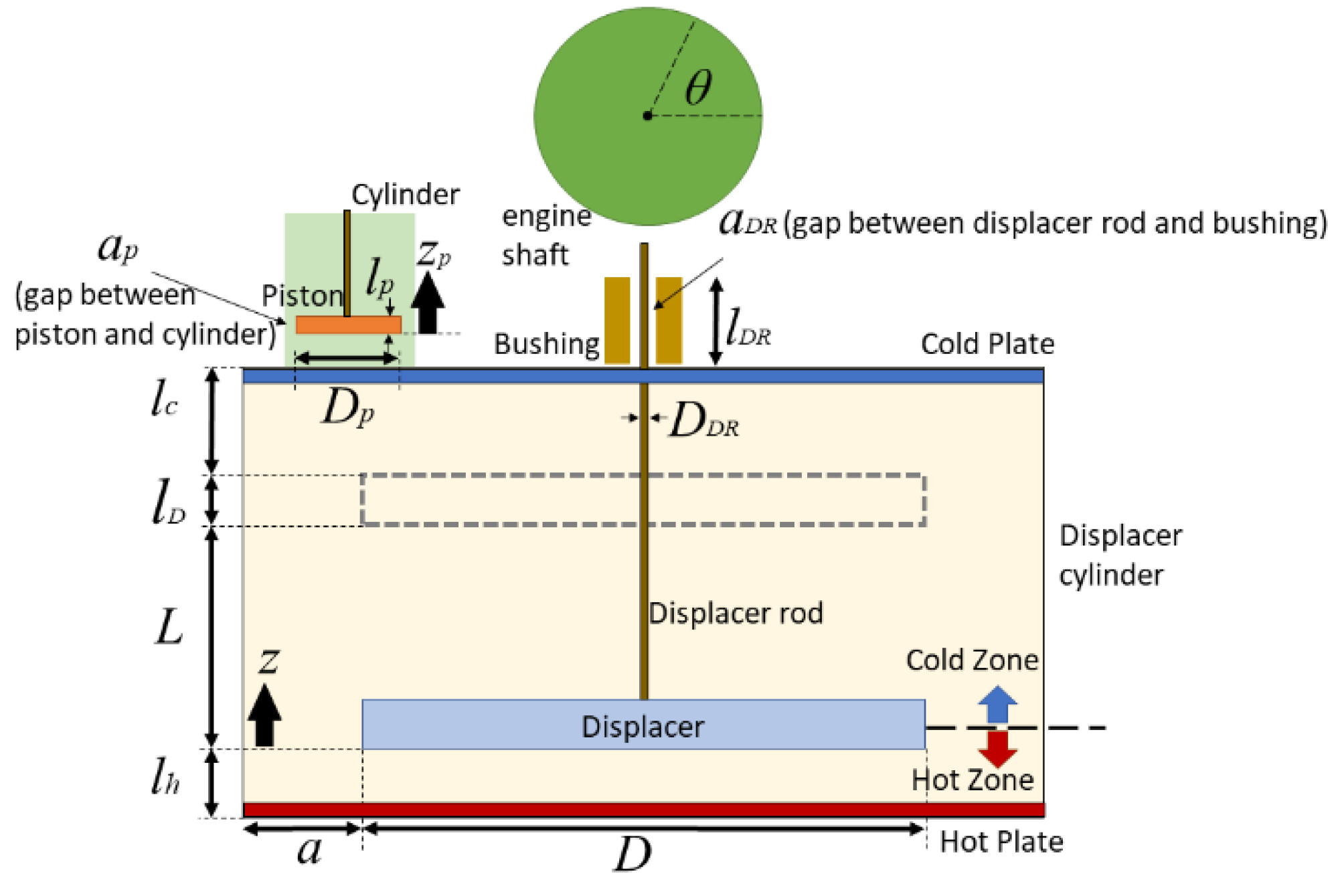
Energies | Free Full-Text | A Theoretical and Experimental Study of Moderate Temperature Alfa Type Stirling Engines | HTML
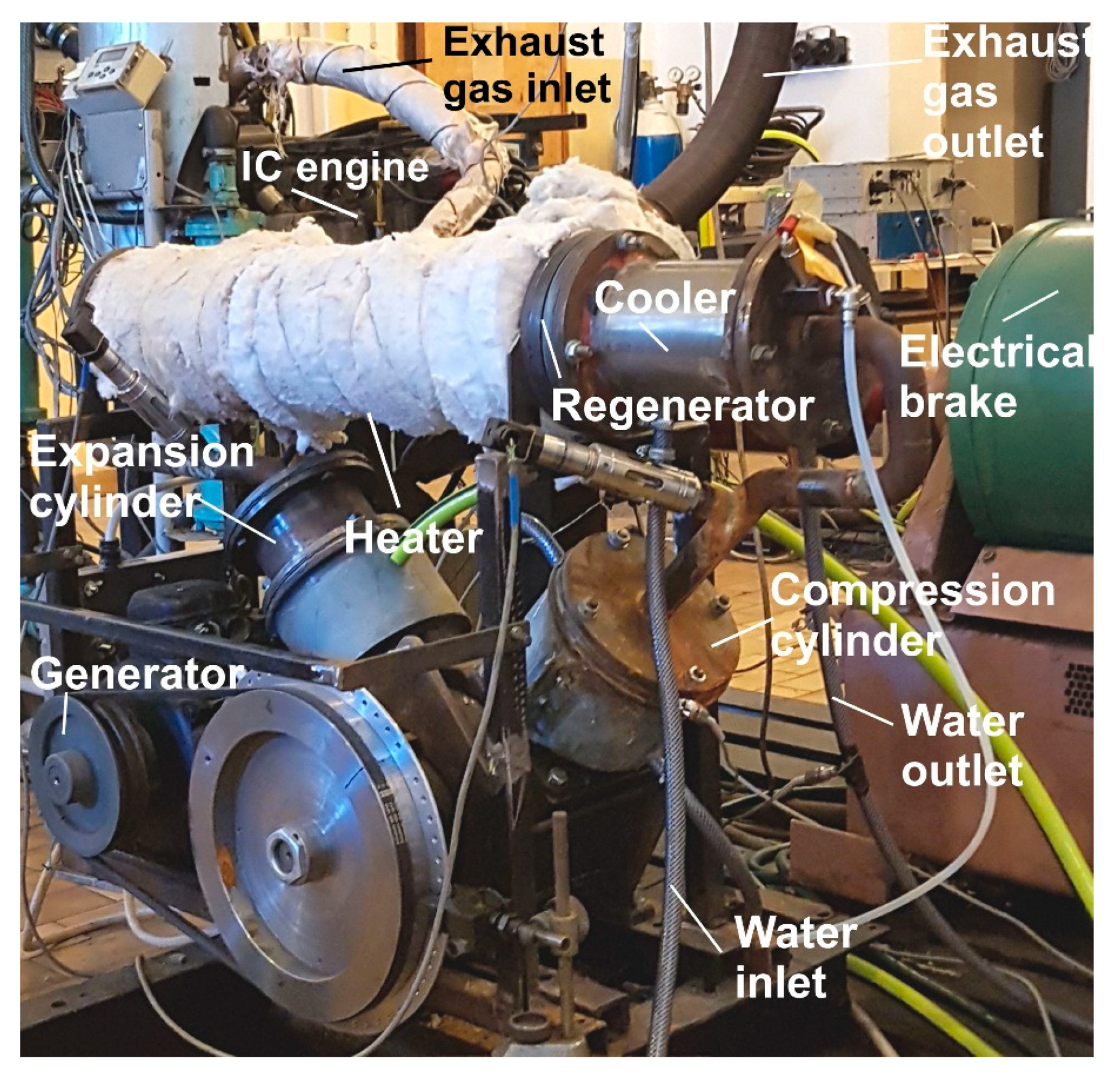
Energies | Free Full-Text | A Theoretical and Experimental Study of Moderate Temperature Alfa Type Stirling Engines | HTML

Energies | Free Full-Text | A Theoretical and Experimental Study of Moderate Temperature Alfa Type Stirling Engines | HTML
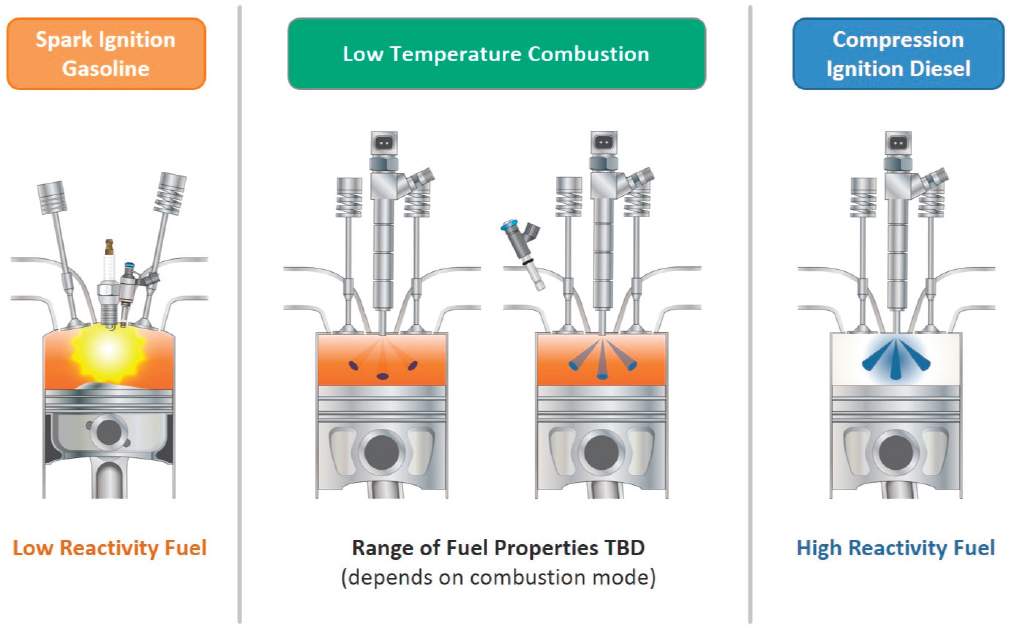
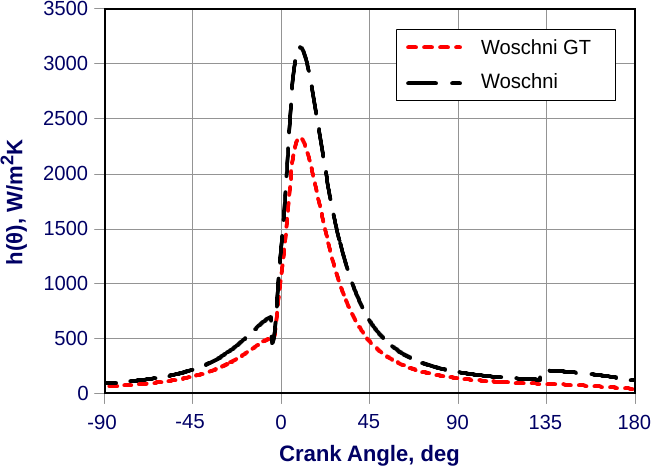
4 Powertrain Technologies | Reducing Fuel Consumption and Greenhouse Gas Emissions of Medium- and Heavy-Duty Vehicles, Phase Two: Final Report | The National Academies Press

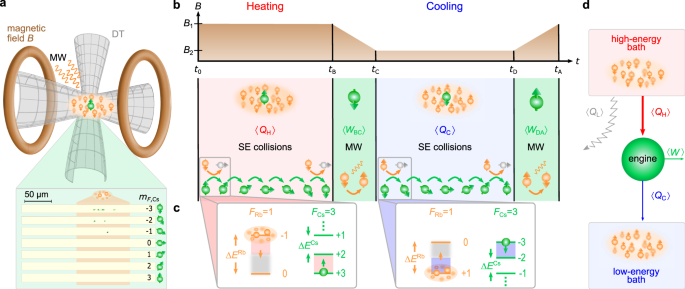
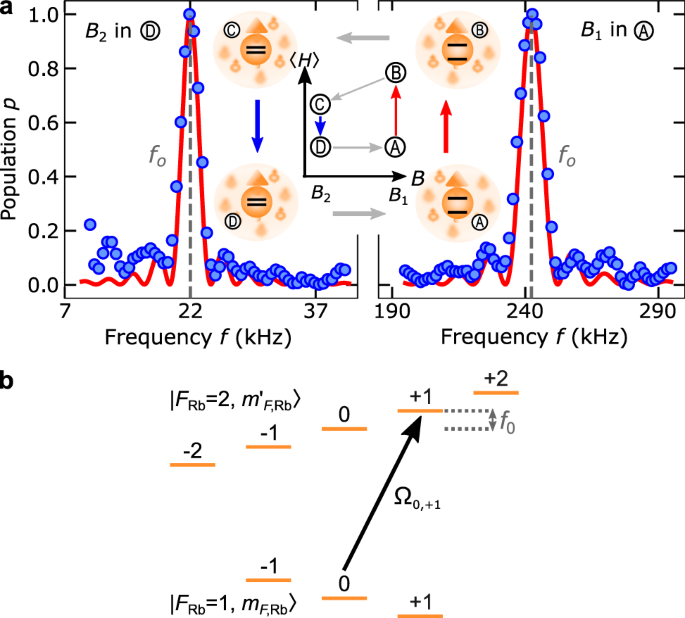
Universality of maximum-work efficiency of a cyclic heat engine based on a finite system of ultracold atoms | Scientific Reports